Welcome To CURA EQMS
Make your Quality Management System easier to comprehend
Simplify QMS elements with digital approach by reducing 80% of complex tasks, making quality your key asset. Integrate with CURA Compliance and Data Assurance into your workflows with CURA.
Solutions
EQMS solutions
Change Control
Any Changes happening at Manufacturing site which would impact the Product Quality & Patient Safety along with all types of Process / Material / Document / Method Changes would be captured under this Change Management as per Regulatory guidance’s.
Deviation Management
To execute systems for documenting and analysing Deviations if products do not meet quality standards and any violations / departures from defined procedures etc.
Audit Management
To streamline processes for planning audits, meeting deadlines, and hitting targets for industry requirements using organized project management and document tracking.
Vendor Qualification
All Raw Materials / Packing Materials which are being used in the Manufacturing Processes shall be Qualified as per the defined procedures and all the procedural steps are covered under this VQ Module.
Market Complaints
Any Customer Complaint received from any source shall be investigated to classify and categorize as per the criticality and same will be investigated to identify the root cause and to implement a Robust CAPA
CAPA Management
To collect and identify issues with product nonconformities so organizations can quickly resolve them and execute preventative strategies.
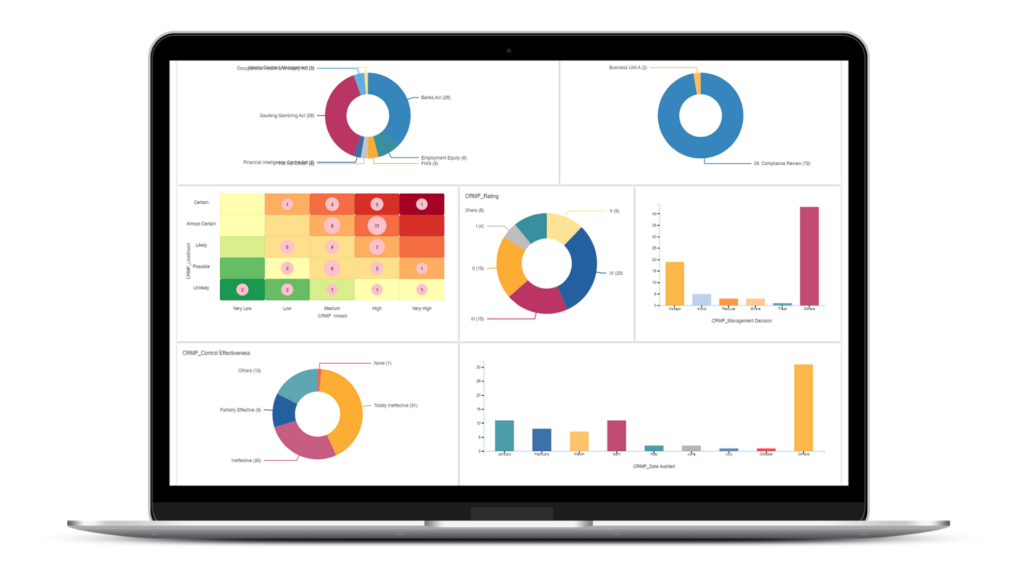
Accurate Business Strategy Insights and Reviews
CURA Data Analytics gives a Clear Insight to take the Right Decisions by the Management for the Overall Effectiveness of QMS at Site.
Features
Easy and Smart Solution for You
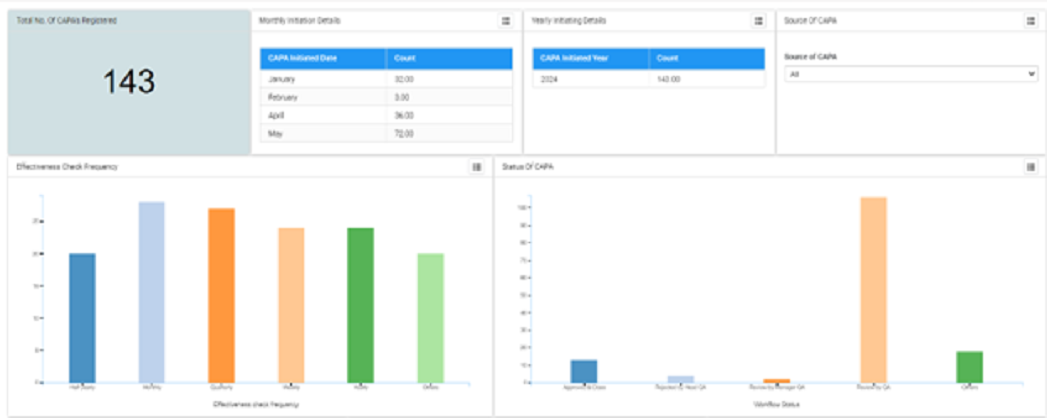
Reporting and analytics
To analyse and measure the effectiveness of quality management efforts that organizations can stay on top of regulatory requirements, audit findings, non-conformance %, customer complaints etc.

Easy Automation
Achieve seamless quality control with automated EQMS solutions.
Data Security
CURA focusses on Data Security. Our EQMS utilizes robust actions to safeguard and shield Vulnerable data, Confirming and guaranteeing that the data is safe and secure and compatible conditions to meet compliance for the Customer’s Crucial Data / Information resources.
Easy Configuration
CURA's EQMS is highly feasible for integration with other modules and can seamlessly connect with ERP systems for smooth operations. Through API integration, customers can enhance and improve their Quality Management Systems (QMS) with CURA's EQMS.
Complying to Global Regulatory Requirements
CURA’s EQMS meets the desired regulatory compliance with stringent regulations namely USFDA 21 CFR Part 11 & EU GMP Annex 11, ALCOA, GMP etc.
Centralized QMS
Centralized QMS Processes (Manage all 6 Quality Processes in a Centralized data Base)

Notifications and Alerts
Proactive Alert Mechanism (Notifications for Approvals / Pop ups for Past Due Actions/ Mail Alerts.
Frequently Ask Questions
EQMS improves quality management by streamlining processes, reducing errors, enhancing traceability, and ensuring consistent application of quality standards across the organization.
While EQMS is often used by large organizations, many solutions are scalable and can be tailored to meet the needs and budget of small and medium-sized businesses.
EQMS automates quality processes by using workflows to handle tasks such as document approvals, CAPA management, audit scheduling, and training tracking, reducing manual effort and the potential for errors.
Data within an CURA EQMS is typically highly secure, with features such as user authentication, role-based access control, data encryption, and regular security audits to protect sensitive quality management information.